
Let's assume there are two (or more) species undergoing charge transfer reactions
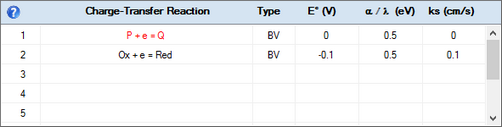
P + e = Q
Ox + e = Red
Let's also assume that the species involved in the first redox couple (written in red) are (strongly) adsorbed on the electrode surface. In such a situation the second charge-transfer process may proceed through the covered and uncovered part of the electrode with a different rate constant. This can be simulated by DigiElch by linking the heterogeneous rate constant of the second CT-reaction to the surface coverage of the species involved in the first redox couple:
1.Click on the Edit command in the Tabbed Window: Simulations and enter the above charge transfer reactions. Activate the Enable Adsorption check box for the first one.
2.Enter values for the standard potential, E° (V), and the heterogeneous rate constant, ks, for both reactions. In the case of the second CT-reaction the value of ks should refer to the charge transfer occurring through the uncovered part of the electrode.
 Note that the adsorbed species might even be electrochemically inactive ones.In this case the heterogeneous rate constant, ks, is simply set to zero.Then the only purpose of the first "redox-couple" is to mimic a partial blockage of the electrode surface for the second (electrochemically active) redox-couple.
Note that the adsorbed species might even be electrochemically inactive ones.In this case the heterogeneous rate constant, ks, is simply set to zero.Then the only purpose of the first "redox-couple" is to mimic a partial blockage of the electrode surface for the second (electrochemically active) redox-couple.
3.Click with the right mouse button on reaction equation involving the the ks-value that is to be linked with the surface coverage of other species. Unlike the example shown above not a simple dialog box but the following property sheet shows up now
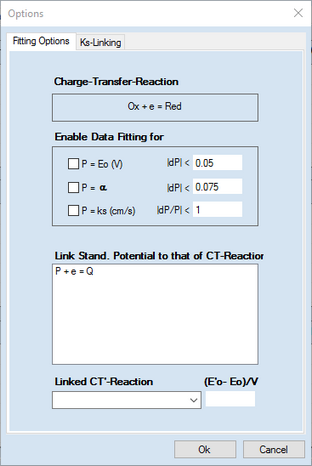
The fist page headlined Fitting Options works exactly in the same way as demonstrated in the above example.
4.Activate the second page headlined Ks-Linking and use the Combo box in the appearing dialog box to select the CT-reaction containing the adsorbed species to which surface coverage the ks - value is supposed to be linked. In the actual example only one CT-reaction comprising adsorbed species is present.
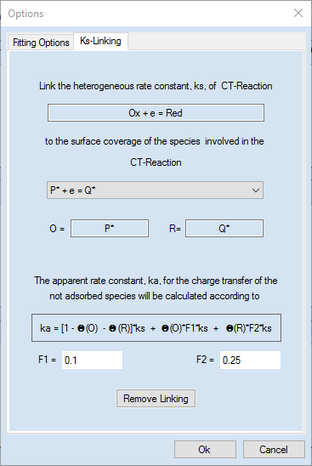
5.Enter values for the factors F1 and F2 and finish with OK.
When proceeding in this way, the second charge transfer process occurs with the standard rate constant, ks, through the uncovered part of the electrode while it proceeds with a standard rate constant ks' = F1*ks and ks'' = F2*ks through the parts of the electrode covered by the species "P" and "Q", respectively.
A heterogeneous rate constant which is linked to the surface coverage of an adsorbed species is plotted in red.
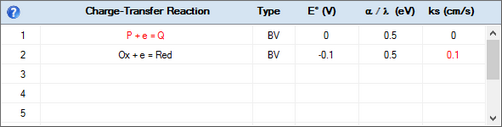
It will be plotted in magenta if the rate constant is additionally selected for being optimized.
